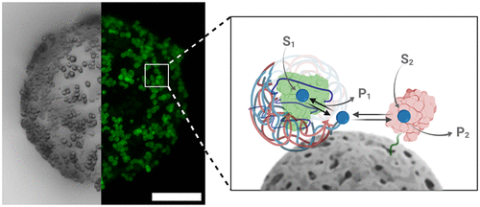
Enzyme compartmentalization is one of the main strategies exploited by nature to create physically separated chemical environments that allow simultaneous enzyme reactions within the cell metabolic networks. However, designing nanostructured architectures that mimic cellular compartments remains a challenge when two competing enzymes must work simultaneously over the same substrate. Herein, we develop a method to fabricate soft hybrids that physically separate two oxidoreductases that compete for NADH with greatly different kinetics. The less competitive enzyme is encapsulated into polymeric capsules capable of recruiting NADH, which are then assembled on porous agarose microbeads where the most competitive enzyme is immobilized. As a result, this functional hybrid enables the simultaneous action of two competing enzymes in the same reaction media, which would otherwise be impossible in a non-compartmentalized system. We demonstrate that substrate recruitment is a powerful approach to building up enzymatic reaction networks with complex dynamics. Moreover, single-particle analysis under operando conditions reveals the impact of enzyme spatial organization on the overall performance of these soft hybrids, underlining the importance of understanding the functional variability within compartmentalized systems. Finally, integrating this compartmentalized system into a model cell-free biosynthetic cascade, we transform vinyl acetate into (S)-β-hydroxybutyrate with a 2 times higher titer than the non-compartmentalized free system. The proposed strategy can be generalized to produce compartmentalized cell-free biosynthetic pathways and multienzyme cascades where enzyme competition is an issue.