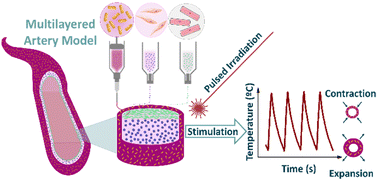
3D-printed cell models are currently in the spotlight of medical research. Whilst significant advances have been made, there are still aspects that require attention to achieve more realistic models which faithfully represent the in vivo environment. In this work we describe the production of an artery model with cyclic expansive properties, capable of mimicking the different physical forces and stress factors that cells experience in physiological conditions. The artery wall components are reproduced using 3D printing of thermoresponsive polymers with inorganic nanoparticles (NPs) representing the outer tunica adventitia, smooth muscle cells embedded in extracellular matrix representing the tunica media, and finally a monolayer of endothelial cells as the tunica intima. Cyclic expansion can be induced thanks to the inclusion of photo-responsive plasmonic NPs embedded within the thermoresponsive ink composition, resulting in changes in the thermoresponsive polymer hydration state and hence volume, in a stimulated on–off manner. By changing the thermoresponsive polymer composition, the transition temperature and pulsatility can be efficiently tuned. We show the direct effect of cyclic expansion and contraction on the overlying cell layers by analyzing transcriptional changes in mechanoresponsive mesenchymal genes associated with such microenvironmental physical cues. The technique described herein involving stimuli-responsive 3D printed tissue constructs, also described as four- dimensional (4D) printing, offers a novel approach for the production of dynamic biomodels.