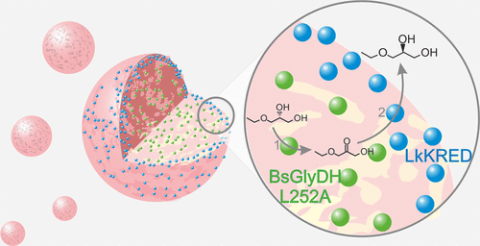
Production of enantiomerically pure molecules is of utmost importance in the pharma industry. In this context, biocatalysis emerges as an alternative to conventional chemical methods due to the exquisite selectivity and specificity underlying the enzymes. In this work, we design a multienzymatic system to perform the deracemization of alkyl glyceryl ethers as potential building blocks for the synthesis of drugs. The key to success in this route is controlling the spatial organization of the enzymes involved in the cascade through their immobilization on porous carriers. By fine tuning the intraparticle organization of an enzymatic cascade comprising an (S)-selective glycerol dehydrogenase from Bacillus stearothermophilus and an (R)-selective ketoreductase from Lactobacillus kefir, we performed the oxidoreductive deracemization of rac-alkyl/aryl glyceryl ethers with a yield up to 100% and enantiomeric excess e.e. > 99%, otherwise impossible using a soluble system. Remarkably, we find that optimal spatial assembly of the biocatalyst ameliorates the inhibition phenomena experimented by the system and increases the deracemization rate by 4-fold. Finally, integrating an enzymatic nicotinamide adenine dinucleotide oxidized disodium salt (NAD+) regeneration system to the heterogeneous biocatalyst, we intensified the process by reusing it in discontinuous and consecutive batch cycles and scaling the reaction up to 250 mM substrate, achieving 100% yield and e.e. > 99%.