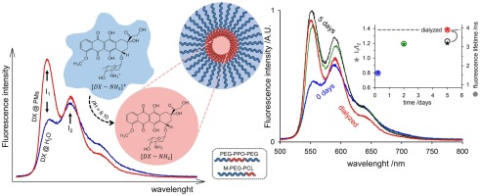
The anticancer drug doxorubicin hydrochloride (DX) shows a high solubility in aqueous media thanks to the positive charge in the ammonium group. This feature, however, affects the drug encapsulation in the hydrophobic domains of polymeric micelles (PMs) used for the targeted delivery of the drug. At basic pH, DX deprotonates but also acquires a negative charge in the phenolic groups of the anthracycline structure. Both the efficiency and the rate of encapsulation will be increased by choosing an appropriate pH such that the drug molecule is in neutral form.