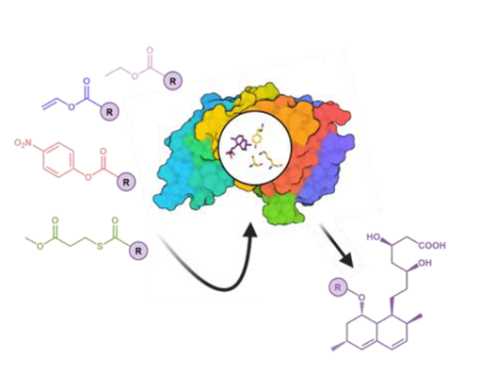
This study identifies new acyl donors for manufacturing statin analogues through the acylation of monacolin J acid by the laboratory evolved acyltransferase LovD9. Vinyl and p-nitrophenyl esters have emerged as alternate substrates for LovD9 acylation mechanism. While vinyl esters can reach product yields as high as the ones obtained by α-dimethyl butyryl-S-methyl-3-mercaptopropionate (DMB-SMMP), the thioester for which LovD9 was evolved, p-nitrophenyl esters display a reactivity even higher than DMB-SMMP for the first acylation step yet the acylation product yield is lower. The reaction mechanisms were elucidated through quantum mechanics (QM) calculations.