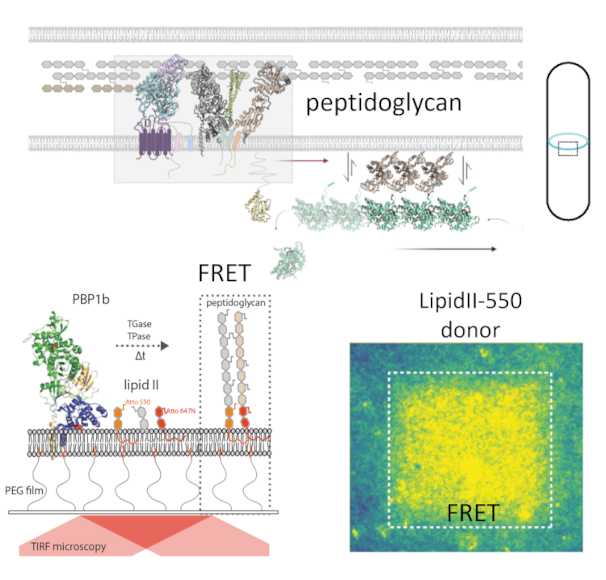
The bacterial life cycle relies on two dynamic and mechanically active assemblies: peptidoglycan and cytoskeleton. The focus of our previous work was to understand the mechanism of peptidoglycan synthesis using real-time microscopy. We developed a novel reconstitution, where a transmembrane cell wall synthase (PBP1b) was inserted in polymer-supported lipid membranes, allowing us to track the 2D dynamics of the enzyme during peptidoglycan synthesis. Using real-time FRET assay, we could monitor glycosyltransferase and transpeptidase activities of Class A PBPs from Escherichia coli, Pseudomonas aeruginosa, and Acinetobacter baumannii. Considering that peptidoglycan is an essential component of the bacterial cell envelope and a direct target of β-lactam and glycopeptides antibiotics, our assay can shed further details on the mechanism of peptidoglycan synthesis and can be adapted for high throughput screening of novel antimicrobials.
PBP1b-647
Related publications:
V. Hernandez-Rocamora*, N. Baranova*, E. Breukink, M. Loose and W. Vollmer (2021) Realtime monitoring of peptidoglycan synthesis by class A PBPs on the lipid membrane. eLife, e61525 *- equal contribution