Cell-free biocatalysis is being increasingly used as a substitute for conventional chemical catalysts, given that enzymes (biological catalysts) are more sustainable and selective in the manufacture of valuable chemicals. Chemical biomanufacturing has benefited from extraordinary advances in molecular and synthetic biology, which have stimulated the creation of new enzymatic cascades (sequences of reactions in which each newly formed product is subsequently transformed into the next).
To increase the output of these enzymatic cascades, biotechnology has succeeded in designing biomolecule-based scaffolds in which multi-enzyme systems are spatially arranged within a few nanometers, thus achieving efficient biosynthetic pathways. However, organizing enzymes with nanometric precision poses a challenge for scaffold design.
Researchers with expertise in protein engineering and heterogeneous biocatalysis at the Center for Cooperative Research in Biomaterials CIC biomaGUNE have developed a nanometrically organized multi-enzyme system that uses engineered proteins known as TRAP proteins as a scaffold for biocatalysis, thus achieving precise control of spatial distribution and physicochemical properties.
The study, published recently by the prestigious scientific journal Nature Communications, “is the first example of a protein scaffold designed to organize several enzymes at the nanoscale and trap reaction intermediates to increase their concentration around the scaffolded enzymes. This had already been demonstrated previously with DNA scaffolds, but never with protein-based ones”, said the Ikerbasque Research Professor Aitziber L. Cortajarena, the center’s scientific director. “We have found that these assembled multi-enzymatic systems have specific productivity up to 5 times higher than non-assembled systems,” confirmed the Ikerbasque Research Professor Fernando López-Gallego, co-author of the study. In addition, they have immobilized this biomolecular scaffold on other solid surfaces, thus creating reusable heterogeneous multifunctional biocatalysts in consecutive reaction cycles.
The research results demonstrate the potential of the TRAP scaffolds developed as spatial organization tools to increase the efficiency of cell-free biosynthetic pathways: “The selected protein scaffold has unique characteristics and provides spatial control at the nanoscale that is unattainable with other conventional protein scaffolds,” they said. This approach makes it possible to manufacture systems with unprecedented control of the parameters that are important in achieving good catalytic performance. So it is an interesting step forward in the transition toward a more sustainable socioeconomic model.
According to the CIC biomaGUNE research professors, “the methodology developed is relatively simple and modular compared with other current approaches. We anticipate that this technology will go a long way toward advancing the manufacture of more stable and efficient multi-enzyme systems. This collaborative work has shown how the combination of protein engineering and biocatalysis has huge potential not only for improving intrinsic catalytic activity and enzyme stability, but also for maximizing the performance of spatially organized multi-enzyme systems. Furthermore, the applications in biocatalysis demonstrated in this work could be extended to other fields of applied science, for example, the integration in energy devices or the formation of biocatalytic materials for various industrial processes.
Bibliographical reference
Alba Ledesma-Fernández, Susana Velasco-Lozano, Javier Santiago-Arcos, Fernando López-Gallego, Aitziber L. Cortajarena
Engineered repeat proteins as scaffolds to assemble multi-enzyme systems for efficient cell-free biosynthesis
Nature Communications
DOI: 10.1038/s41467-023-38304-z
Figure 1: Illustration of a protein scaffold (pink, light blue and purple) on which three enzymes (blue, green and red) are assembled. These are spatial organization tools to increase the efficiency of an enzymatic cascade (S: substrate. I: intermediate. P: product). (Source: CIC biomaGUNE)
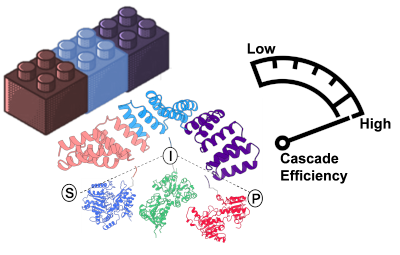
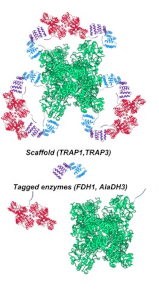
Figure2: Schematic representation of a protein scaffold formed by the TRAP1 (in light blue) and TRAP3 (in purple) proteins on which the FDH1 (in red) and AlaDH3 (in green) enzymes are assembled. (Source: CIC biomaGUNE)