A collaboration between ALBA Synchrotron PhD student and researcher Johannes Groen and Eva Pereiro and the groups of Aitziber L. Cortajarena (CIC biomaGUNE) and Ana V. Villar (IBBTEC, specialised in fibrosis) have visualised for the first time in 3D the exact location and how a nanomaterial drug behaves in whole cells. This drug is a therapeutic protein-nanomaterial hybrid specifically designed to inhibit the collagen overproduction after a myocardial fibrosis event. CIC biomaGUNE researchers have designed and prepared this novel nanomaterial.
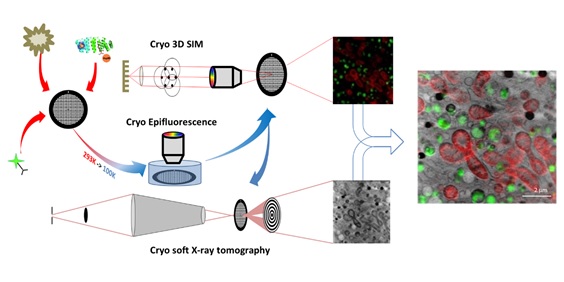
Using a new approach, ALBA Synchrotron researchers combined cryo-3D correlative fluorescent light and X-ray tomography to locate, for the first time, the nanomaterial and to obtain high resolution information on the cellular morphological changes after treatment, proving the antifibrotic properties of the drug.
First, the intracellular location allows to understand what possible routes are used to enter the cell, secondly, how the cell deals with a specific agent and finally, what are the effects induced by the treatment on the structural morphology of the cells.
The results obtained in this study pave the way for the introduction of nanomaterial-based drugs and nanomedicine into the clinic as they reinforce the usefulness of imaging techniques for evaluating cellular structure after the application of specific treatments.
Combination of techniques and beamlines
The samples were prepared at the ALBA Synchrotron and travelled to the B24 beamline at the Diamond Light Source (UK) to acquire 3D fluorescent data using their cryo-3D structured illumination microscopy (cryo-3D-SIM) setup. Then, at the MISTRAL beamline of ALBA, a cryo soft X-ray tomography (cryo-SXT) was performed, which allowed to study whole cells without the need to stain or manipulate the samples.
In addition, the full workflow in cryogenic conditions maximized efficiency by maintaining sample integrity and quality between both experiments, which was crucial to locate for the first time a protein-nanomaterial hybrid in whole cells.
Cryo-3D-SIM confirmed the presence of the therapeutic agent in treated cells, detecting it through its fluorescence signal, whereas cryo-SXT revealed the ultrastructural environment and subcellular localization of the nanomaterial with high spatial correlation accuracy.
The MISTRAL beamline is currently in the process of setting up its own cryo-3D-SIM system, which will allow future users to perform all the process at the ALBA Synchrotron.
Cardiac fibrosis
Cardiac fibrosis is a health condition that negatively influences the progression of many heart diseases and affects millions of people worldwide. It promotes the pathological increase of collagen deposition at the site of injury and the production of a scar, which can result in the disruption of tissue function in the worst cases.
The most common causes of cardiac fibrosis are ischaemia, infarction, cardiomyopathies and myocarditis, which require short- and long-term follow-up due to pump dysfunction and myocardial stiffness. These pathologies are closely related to an unhealthy diet or lifestyle, advanced age and stress, among others.
There is no preventive or curative treatment for cardiac fibrosis and, although a number of therapeutic agents prescribed for other cardiovascular disorders have been shown to exert beneficial effects on myocardial fibrosis, in most cases an invasive surgery to save the patients live is needed.
Reference: Johannes Groen, Ana Palanca, Antonio Aires, Javi Conesa, David Maestro, Stefan Rehbein, Maria Harkiolaki, Ana Victoria Villar, Aitziber L. Cortajarena and Eva Pereiro. Correlative 3D cryo X-ray imaging reveals intracellular location and effect of designed antifibrotic protein-nanomaterial hybrids. Chemical Science (2021). DOI: https://doi.org/10.1039/D1SC04183E
With the collaboration of Fundación Española para la Ciencia y la Tecnología. The ALBA Synchrotron is part of the Unidades de Cultura Científica y de la Innovación (UCC+i) of the FECYT and has received support through the FCT-20-15798 project.