- Development of glycomimetics as inhibitors for the treatment of cancer, autoimmune diseases and allergy
- Development of anti-glycan vaccines
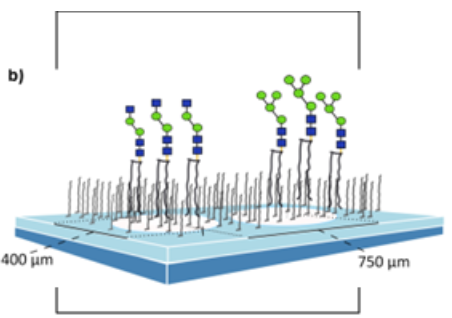
Binding specifities of C-type lectin receptors
Myeloid C-type lectin receptors (CLRs) expressed by antigen-presenting cells are pattern recognition receptors involved in the recognition of pathogens as well as self-antigens. The interaction of carbohydrate ligands with a CLR can shape immune responses. Although several CLR ligands are known, there is still a lack of knowledge on CLR targeting using carbohydrate ligands. This is mainly due to the weak affinity of lectin-carbohydrate interactions often rendering a multivalent carbohydrate presentation necessary. We analysed the impact of a multivalent presentation of the trisaccharide Lewis X (LeX) epitope on its interaction with the CLR Macrophage galactose-type lectin-1 (MGL-1). Glycan arrays including N-glycan structures with terminal LeX were prepared by the enzymatic extension of immobilized synthetic core structures with two recombinant glycosyltransferases. Incubation of arrays with a MGL-1-hFc fusion protein showed an up to 10-fold increased binding to multiantennary N-glycans displaying LeX structures compared to the monovalent LeX trisaccharide. Multivalent presentation of LeX on the model antigen ovalbumin (OVA) led to increased cytokine production in a dendritic cell (DC)/T cell co-culture system. Furthermore, immunization of mice with the LeX-OVA conjugates modulated cytokine production and the humoral response compared to OVA alone. This study provides inside in how multivalent carbohydrate-lectin interactions can be exploited to modulate immune responses. (Serna et al. 2014)
The synthesis of a collection of 33 xylosylated and core-fucosylated N-glycans found only in non-mammalian organisms such as plants and parasitic helminths has been achieved by employing a highly convergent chemo-enzymatic approach. The influence of these core modifications on the interaction with plant lectins, with the human lectin DC-SIGN (Dendritic Cell-Specific Intercellular adhesion molecule-3-Grabbing Non-integrin), and with serum antibodies from schistosome-infected individuals was studied. Core xylosylation markedly reduced or completely abolished binding to several mannose-binding plant lectins and to DC-SIGN, a C-type lectin receptor present on antigen presenting cells. Employing the synthetic collection of core-fucosylated and core-xylosylated N-glycans in the context of a larger glycan array including structures lacking these core modifications we were able to dissect core xylose and core fucose specific anti-glycan antibody responses in S. mansoni infection sera, and we observed clear and immunologically relevant differences between children and adult groups infected with this parasite. The work presented here suggests that, quite similar to bisecting N-acetylglucosamine, core xylose distorts the conformation of the unsubstituted glycan, with important implications for the immunogenicity and protein binding properties of complex-N-glycans. (Brzezicka et al. 2015)